Gas chromatography - mass spectrometry
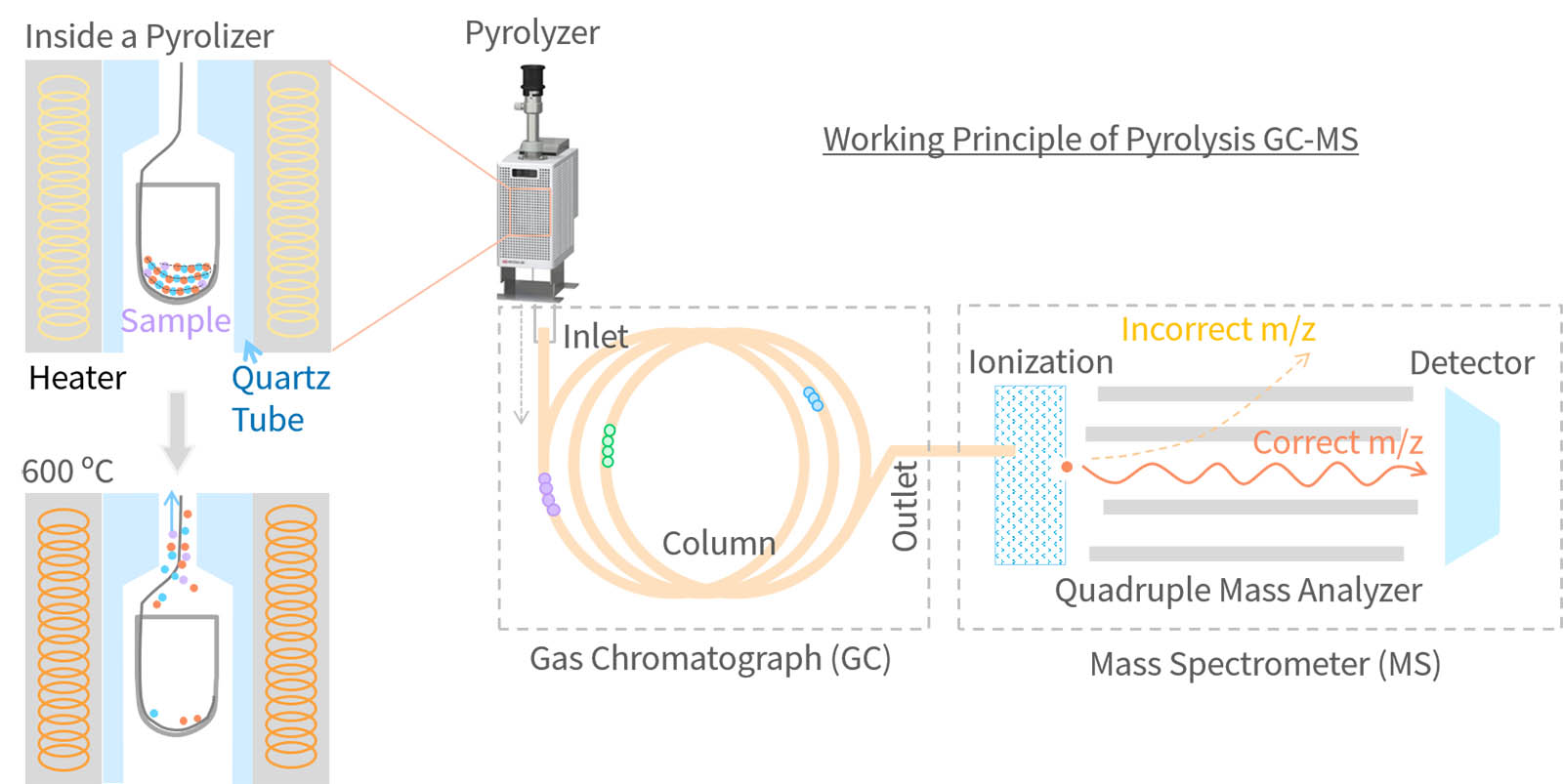
Gas chromatography - mass spectrometer (GC-MS) can effectively separate mixtures of compounds in the vapor phase and identify each phase through mass fragments. Since the GC column ovens can be heated in excess of 300 °C, the term ‘gas’ refers to anything that can be vaporized at those temperatures, including solvents, oils, and even waxes. However, solid materials with high molecular weights (e.g. polymers) cannot be made volatile at these temperatures, so GC-MS itself could not analyze polymeric materials.
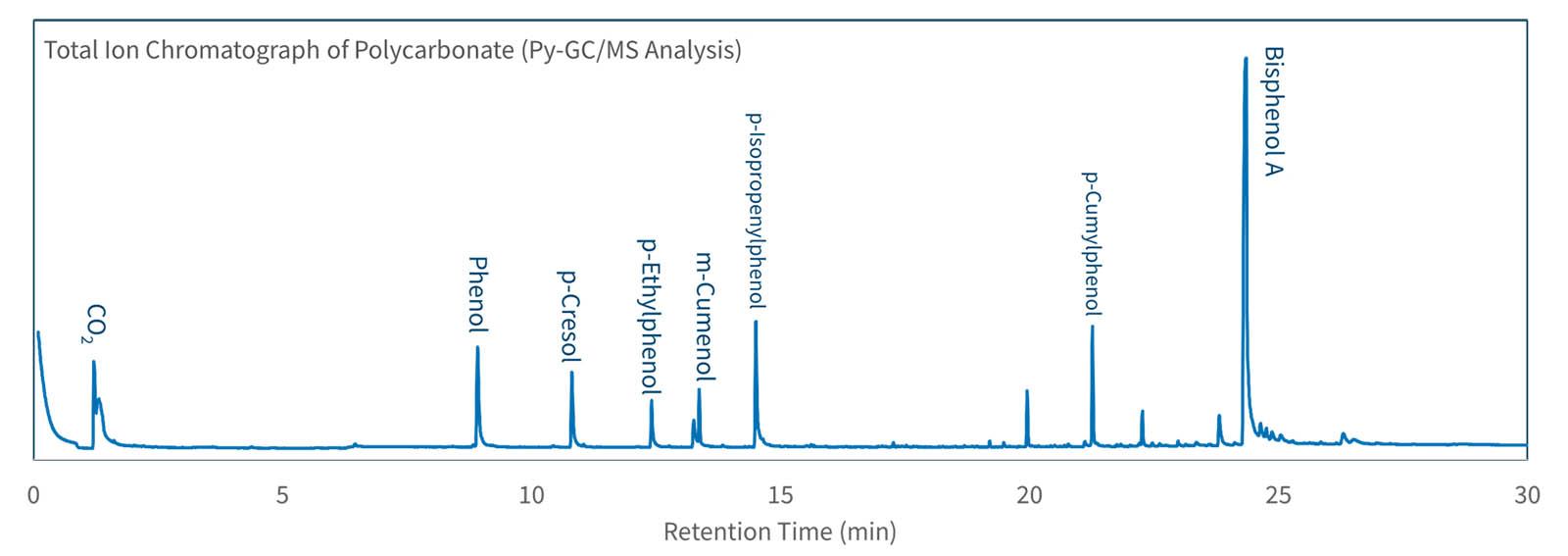
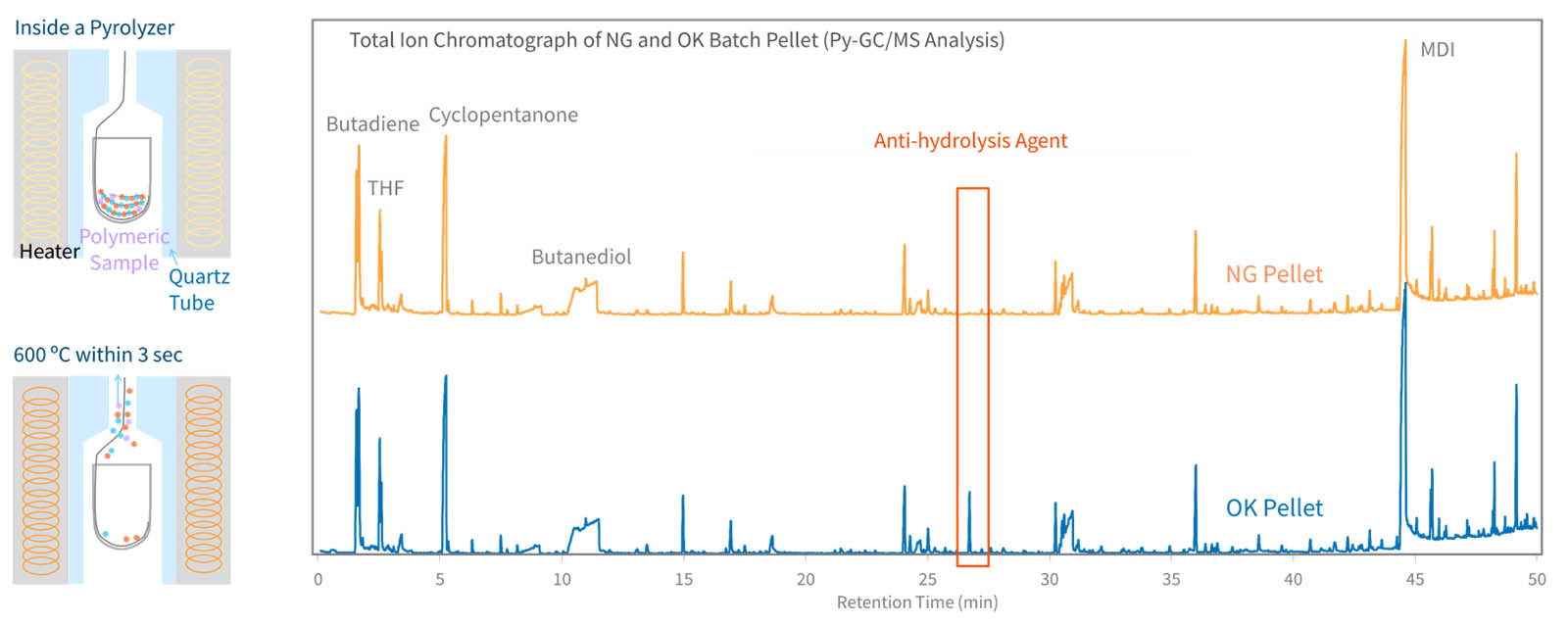
Pyrolysis GC-MS Analytical pyrolysis extends the use of GC–MS by creating volatile compounds from nonvolatile solids like polymers. When heated to temperatures sufficiently high to cause bond dissociation, polymers and other macromolecules degrade reproducible and generate a characteristic pyrogram of the original material. The resulting pyrolysis products retain structural information from the original sample, permitting the identification of pure materials, blends, and copolymers.